

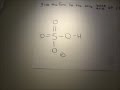

















Solved: What is the conjugate acid for NH2-? a. N3- b. NH2- c. NH4+ d. H+ By signing up, you'll get thousands of step-by-step solutions to your... Answer to Give the formula for the conjugate base of each acid. a. HNO2 b. NH4+ c. H202l... Whats the formula for NH4 + SO32-+1. Answers (1) Gabriel Mitchell 17 January, 10:10. 0. I believe NH4 is ammonium SO3 2 - is sulfite is that what you were asking for. Comment; Complaint; Link; Know the Answer? Answer. Not Sure About the Answer? Find an answer to your question 👍 “Whats the formula for NH4 + SO32- ...” in 📗 Chemistry if the answers seem to be not correct or there’s ... Problem: Give the conjugate base of the following Bronsted-Lowry acid: NH4+. FREE Expert Solution. 79% (93 ratings) Problem Details. Give the conjugate base of the following Bronsted-Lowry acid: NH 4 +. Learn this topic by watching Conjugate Acids and Bases Concept Videos. All Chemistry Practice Problems Conjugate Acids and Bases Practice Problems. Q. Label each of the following as being a ... Conjugate acid/base pairs means that one substance (the base) is the proton (H+) acceptor, and the other, the acid, is the proton donor. In an acid/base pair, the structures of the two compounds should be almost exactly the same, except the acid should have one more H than the base. Write the formula of the conjugate base for acid NH4+? Answer Save. 2 Answers. Relevance. sakshi. 6 years ago. nh3 is the conjugate base for the acid nh4+.....as nh4+ will donate h+ it will act as an lowry bronsted acid.....after donating h+,it will form nh3, the conjugate base of ammonium.... 0 0. Viictoory. 6 years ago. NH4+ + H2O --> NH3 + H3O+ So NH3 is the conjugate base of NH4+ 1 0 ... NH_3 NH_4^+ + H_2O -> NH_3 + H_3O^+ NH_4^+ is the acid because it donates an H^+ ion to the water. It then becomes ammonia (NH_3), which would be the conjugate base of NH_4^+. And the conjugate acid of bisphosphate ion, HPO_4^(2-), is H_2PO_4^-. As with all of these conjugate acid/conjugate base problems, I am simply simply exchanging a proton, H^+, and conserving mass AND charge. If mass and charge were not conserved, it would not be a realistic exercise. What are the conjugate bases of H_2SO_4, HSO_4^-, NH_3, HO^-, and NH_2^-? What are their conjugate acids? Question: Give The Formula Of The Conjugate Base Of HSO4- . Give The Formula For The Conjugate Acid Of HSO4-. This problem has been solved! See the answer. Show transcribed image text. Videos. Step-by-step answer 04:16 0 0. Expert Answer 100% (54 ratings) Previous question Next question ... Give the formula of the conjugate base of each of the following. (Type your answer using the format CO2 for CO2, (NH4)2CO3 for (NH4)2CO3, [NH4]+ for NH4+, - 15415538
[index] [8227] [8893] [5859] [4026] [780] [203] [4484] [224] [773] [7161]
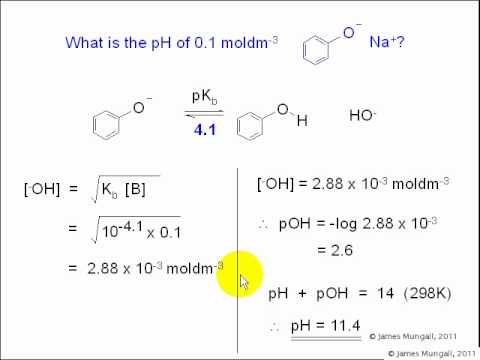
Here I am just answering one of my hw problems. I will try to post as much hw as I can This is for an OCHEM CLASS. All the answers provided are checked by th... http://www.chemistry.jamesmungall.co.ukAcid Base Chemistry7. Relative acidity and basicity -- competition for H+ a. pKa and pKb of conjugate acids and bases[... Here you will find curriculum-based, online educational resources for Chemistry for all grades. Subscribe and get access to thousands of top quality interact... In the Brønsted-Lowry definition of acids and bases, a conjugate acid-base pair consists of two substances that differ only by the presence of a proton (H⁺).... Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs.More free chemistry help at www.chemistnate.com Add some acid. Add some conjugate base. What's the pH? This is the old-school way. You can also use the Henderson-Hasselbalch Equation. In this example I use... Thanks to all of you who support me on Patreon. You da real mvps! $1 per month helps!! :) https://www.patreon.com/patrickjmt !! Example 2: https://www.you... Finding the equation of a Polynomial from a graph by writing out the factors. This example has a double root. I show you how to find the factors and the lead... 🚀To book a personalized 1-on-1 tutoring session:👉Janine The Tutorhttps://janinethetutor.com🚀More proven OneClass Services you might be interested in:👉One... Calculating mass of the salt needed for a buffer solution.
Copyright © 2024 m.livesports24.site